TEM Testing for Virus & Vector Characterization
GMP-certified TEM testing viral vector characterization
Table Of Content
TEM Testing for Virus & Vector Characterization
Cell and gene therapy (CGT), also referred to as advanced therapy medicinal products (ATMPs) by EMA, utilizes gene delivery vehicles to introduce therapeutics directly into patients or to modify cells (e.g., TIL, iPSCs, CAR T-cells) for the treatment or prevention of diseases. We offer GMP-certified TEM testing for viral vectors such as lentivirus, retrovirus, adenovirus, and adeno-associated virus (AAV) often used for such approaches due to their modifiability.
We provide GMP-compliant TEM testing for AAVs and other viral vectors, meeting the stringent requirements set by regulatory authorities such as EMA, FDA, and BP. Our TEM testing service includes the determination of capsid particle population, assessment of capsid integrity and purity, and distinguishing between different recombinant AAV (rAAV) serotypes (e.g., rAAV9, rAAV8, rAAV2). Our method is validated against orthogonal methods (e.g., CDMS, MP, AUC) using multiple rAAV serotypes (rAAV9, rAAV8, rAAV2), ensuring precision and robustness. Additionally, the low sample requirements (2 µL, 1×10¹¹ vp/mL) combined with rapid turnaround times enhances the efficiency of AAV commercialization. Our nsTEM method is robust and accurate across a range of 5×10¹⁰-1×10¹³ capsids/mL and not influenced by the tropism of rAAVs. From a single sample, nsTEM can determine transgene incorporation (full-empty ratio), detect aggregates and debris, assess morphology, and evaluate capsid integrity. This comprehensive analysis ensures that all critical quality attributes of the rAAV particles are thoroughly characterized.
Negative staining TEM – a fast and cost-effective solution for AAV characterization qualification
The image below demonstrates the advantages of negative staining TEM (nsTEM) over traditional methods like CDMS, MP, AUC or CryoTEM in terms of low sample volume, speed, and cost-effectiveness.
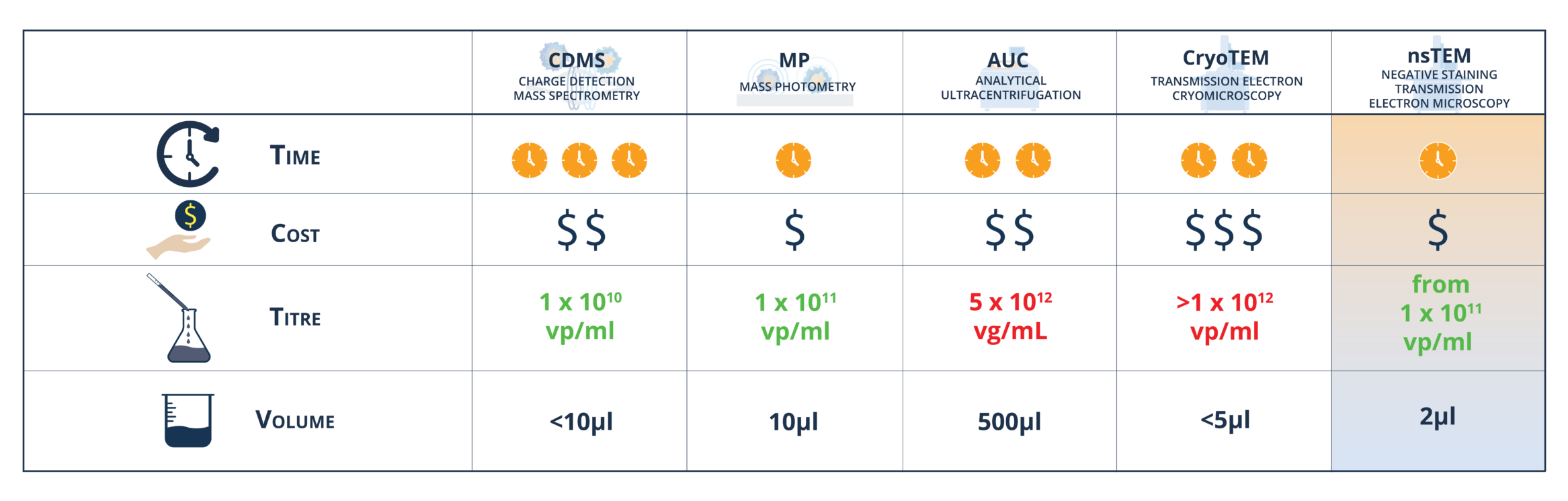
Low sample requirements:
nsTEM requires minimal sample volumes (2 µL) with a concentration of 1×1011 viral particles per milliliter (vp/mL), making it a sustainable and cost-effective alternative compared to conventional methods like AUC, CDMS, or MP.
Speed and efficiency:
nsTEM provides rapid results, facilitating timely decision-making without the delays associated with other methodologies. In contrast to techniques such as CDMS, MP, or AUC, nsTEM offers faster turnaround times, delivering outcomes within a few business days.
Simplicity and cost-efficiency:
nsTEM demands minimal sample preparation, offering a streamlined and cost-effective approach compared to other methods that involve complex setups and expensive procedures. Furthermore, nsTEM provides competitive timelines and pricing relative to CDMS, AUC or CryoTEM.
Visualization of rAAV particles via nsTEM
Understanding the intricate structure of rAAV particles is essential for developing effective gene therapies. The following image visualizes rAAV particles using negative staining TEM (nsTEM), allowing for an in-depth analysis of the capsid particle population. This includes accurate characterization of both the viral load and capsid integrity.
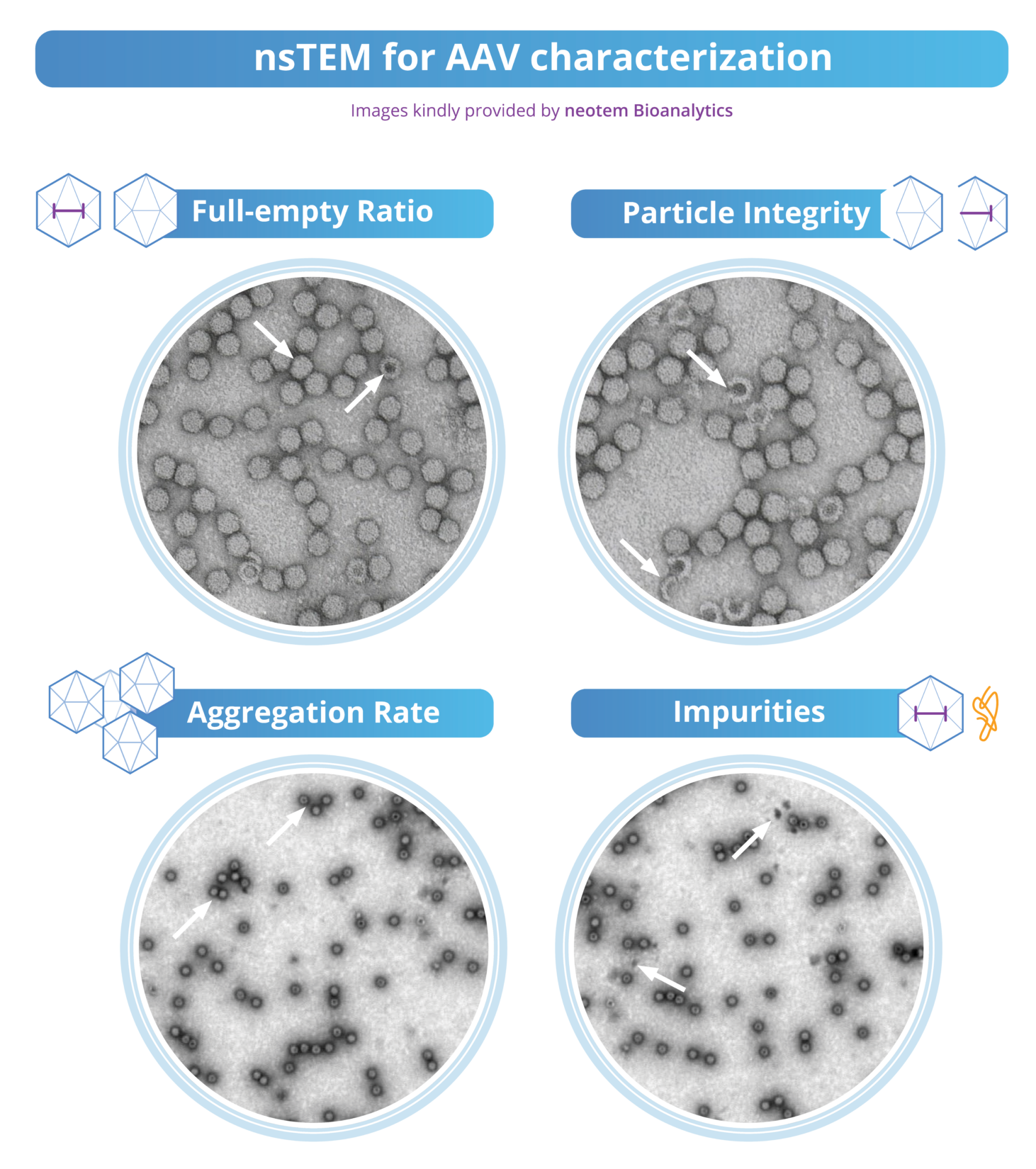
In the image above, AAV particles are shown using negative staining TEM. 2 µL of AAV material (or other viral vectors) is applied to a TEM grid and embedded in a heavy metal salt solution (e.g., uranyl acetate). This solution serves as a contrasting agent by deflecting electrons due to its high electron density. In this imaging process, full AAV particles appear bright against a dark background. The heavy metal stain surrounds the particles but does not penetrate them. Consequently, areas with heavy metal salts scatter more electrons than those with viral particles embedded in the stain. This scattering effect results in the observed contrast, with the heavy metal stain creating a dark background while the AAV particles remain bright.
Empty particles are darker internally because the stain infiltrates and fills their inner cavities. These particles either contain the stain or are collapsed, with the stain deposited on their surface. This increased electron scattering results in a darker appearance inside these particles. By counting the particles, the full-empty ratio can be determined. This ratio is important for assessing the quality of the AAV product, as a higher ratio indicates a greater proportion of functional capsids, which is essential for effective gene therapy.
Intact capsids appear uniformly bright, whereas damaged capsids display irregular shapes, fragmentation, missing portions, or uneven staining due to disrupted internal contents. This contrast facilitates detailed imaging and assessment of capsid integrity. Aggregates formed by clustered damaged capsids and cellular debris are also visible, indicating compromised structural integrity. Additionally, nsTEM can detect potential contaminants and impurities in products like rAAVs, including viral particle aggregates, residual cellular debris from host cells, empty capsids lacking encapsidated DNA, or other viral particles.
Evaluating method comparability of nsTEM
To meet the stringent requirements for GMP-compliant rAAV release, our nsTEM method has been refined. Comparability studies with orthogonal methods such as CDMS, MP, and AUC confirm that our enhanced nsTEM method is both reliable and robust. Rigorous testing has shown no alterations in results due to particle staining or aggregation from the staining process. Additionally, direct comparisons with these orthogonal methods have demonstrated that nsTEM provides precise and consistent particle characterization, ensuring both accuracy and reliability. In addition, our nsTEM method is validated using multiple rAAV serotypes (rAAV9, rAAV8, rAAV2) to guarantee precision and robustness.
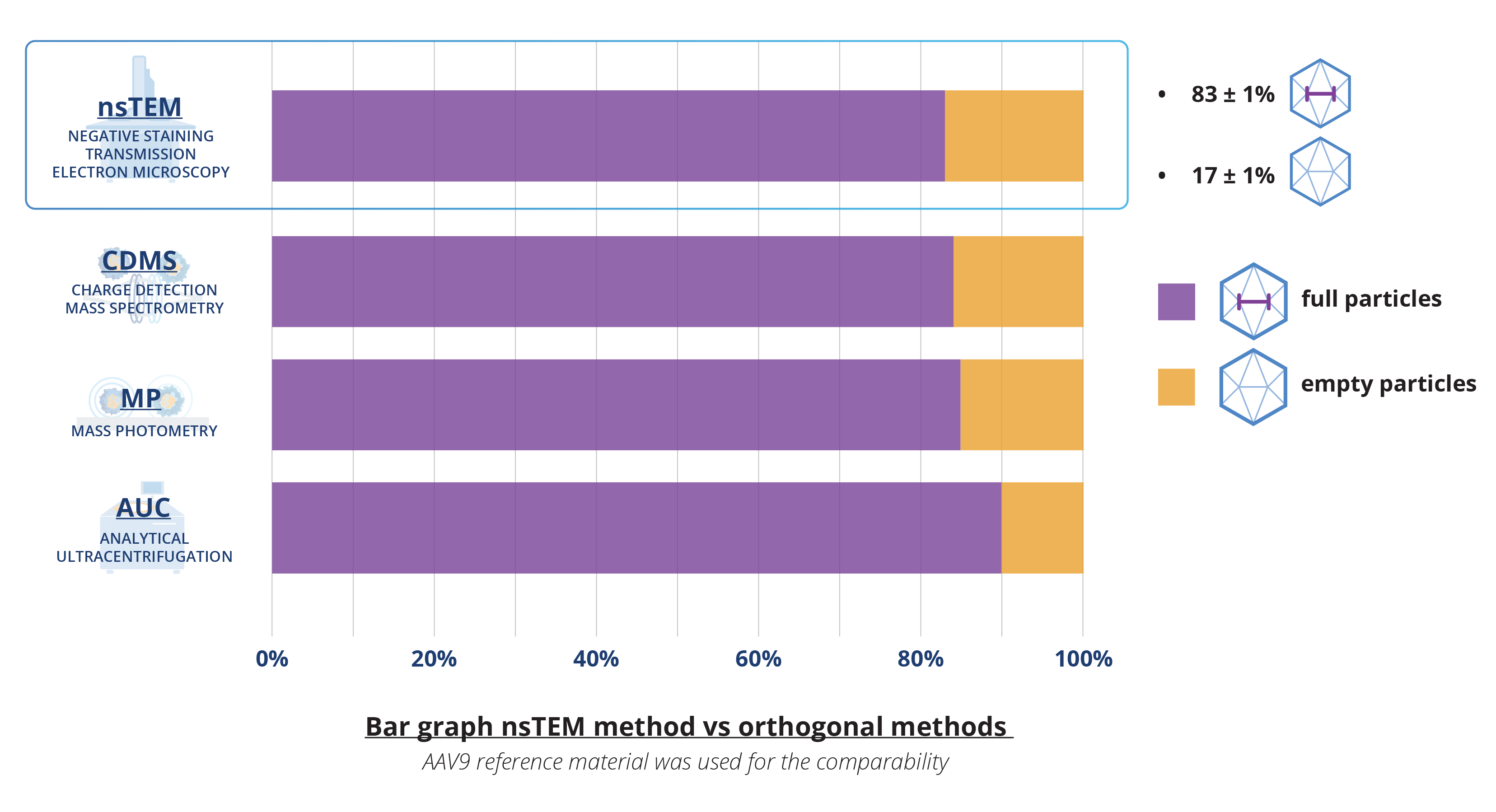
Advantages of nsTEM for AAV characterization
According to current guidelines for assessing the quality of AAVs, multiple analytical methods are recommended for characterizing the capsid particle population in rAAV products. Consequently, selecting from a wide array of tools for AAV characterization can be overwhelming.
Our enhanced nsTEM method has been confirmed as both reliable and robust through comparability studies with orthogonal methods such as CDMS, MP, and AUC. Direct comparisons with these methods have demonstrated that nsTEM provides precise and consistent particle characterization, ensuring accuracy and reliability. Furthermore, our nsTEM method is validated using multiple rAAV serotypes (rAAV9, rAAV8, rAAV2) to guarantee precision and robustness.
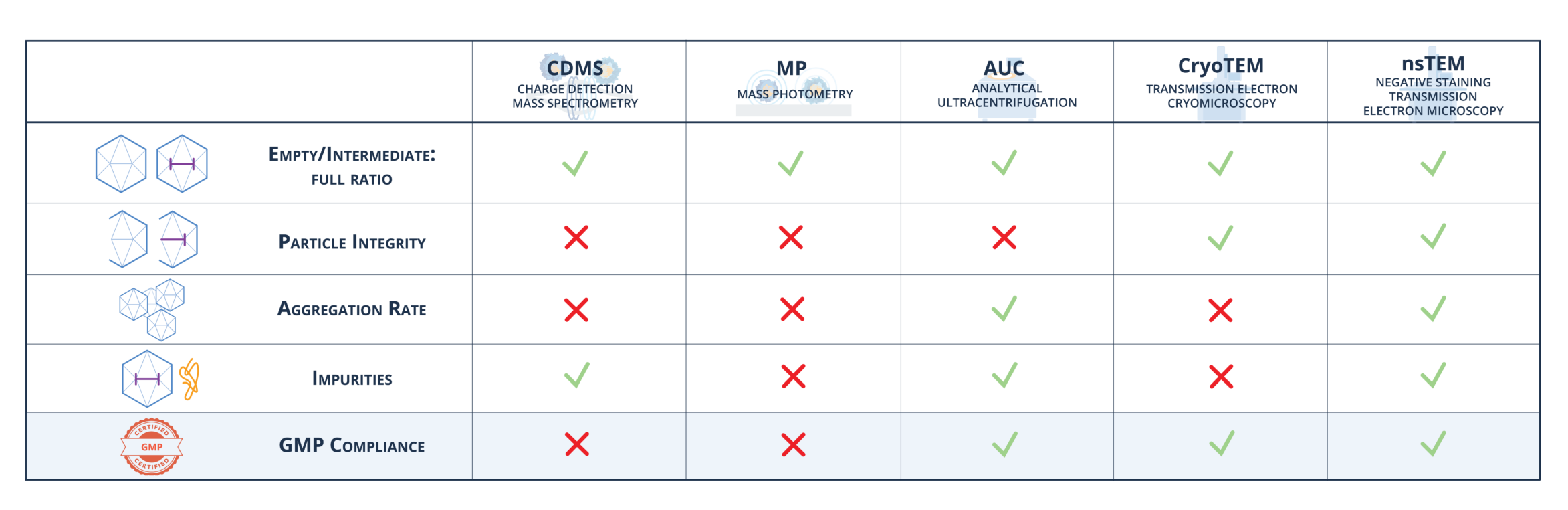
GMP-certified AAV characterization:
nsTEM offers full GMP-compliant characterization of AAVs and provides thorough analysis, including precise determination of the full-empty ratio, all without the need for buffer exchange or dilution. nsTEM can analyze samples from every production step and accurately classify particles, unaffected by proteins, cell debris, or aggregation that can impair other methods like MP. nsTEM allows for accurate assessment of particle integrity and detection of aggregation rates. Unlike CDMS, MP or AUC, which may not fully offer this capability, nsTEM provides a reliable technique for comprehensive particle analysis.
Robust validation & GMP compliance:
Our TEM testing method undergoes rigorous validation against industry standards such as CDMS and AUC, ensuring both reliability and precision. TEM provides visual insights and offers both quantitative and qualitative data, enabling comprehensive analysis of biologic products, including recombinant proteins, mAbs, and CGT products (e.g., CAR-T cells, AAVs, Lentivirus, VLPs). nsTEM offers many advantages over other methods like CDMS, MP, and ultracentrifugation (AUC) in delivering detailed structural information, thereby ensuring product quality and safety. Our TEM testing is GMP-compliant for batch release, unlike CDMS and MP. For MP, sample impurities interfere with the analysis, resulting in low robustness and non-compliance with GMP standards.
Applications of TEM across different products:
Whether for process development analysis or conducting CMC testing of recombinant proteins, mAbs, and cell CGT products (e.g., cell therapies, iPSCs, AAV, lentivirus), TEM plays a crucial role in advancing biopharmaceutical research and development. Regulatory authorities recommend its use for viral safety testing and the characterization of viruses and vectors in ATMPs.
Let´s break down some FAQs!
Why is AAV characterization so important?
Characterizing rAAV-based therapies involves an in-depth analysis of the capsid particle population, including precise assessment of the viral load and capsid integrity. Throughout AAV production, particles lacking genetic material are generated alongside the desired capsids carrying the target transgene. Empty and partially filled AAV particles could diminish treatment efficacy by decreasing the delivery of therapeutic genetic material to target cells, potentially eliciting an immune response. Negative staining TEM allows for a comprehensive AAV assessment and an in-depth analysis of the capsid particle population.
What are the critical quality attributes of rAAVs?
Critical quality attributes (CQAs) of gene therapy vectors encompass various parameters crucial for their efficacy, safety, and regulatory compliance. Understanding and controlling these attributes are imperative to ensure reproducibility and consistency of gene therapy products. In the production of rAAVs, attributes such as virus titer, capsid content, and aggregation are identified as key CQAs that directly impact the potency, purity, and safety of rAAV-mediated therapies. These attributes are essential for the optimal performance of gene therapy vectors and form a critical part of the comprehensive CMC testing. We provide GMP-compliant TEM testing for AAVs, meeting the stringent requirements set by regulatory authorities.
What are the key advantages of AAV vectors?
Recombinant AAV (rAAV) vectors offer several advantages over other vector systems, such as transduction of terminally differentiated and non-dividing cells, low pathogenicity and immunogenicity, stable transgene expression, and potential targeted integration. These unique biological and biophysical properties enable AAVs to be utilized in a wide range of clinical applications across multiple diseases.
Regulatory guidelines to follow:
- EMA Guideline on the Quality, Non-clinical and Clinical Aspects of Gene Therapy Medicinal Products: EMA/CAT/80183/2014.
- FDA Guidance for Industry: Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs).
- British Pharmacopoeia on Advanced Therapy Medicinal Products Guidance: Characterisation of the Capsid Particle Population in rAAV Products.
If you’re interested in learning more, don’t hesitate to reach out
