TEM Testing for Adventitious Agents & RVLPs
TEM testing for viral safety evaluation
Table Of Content
TEM Testing for Adventitious Agents & Retrovirus-like Particles
TEM testing plays an important role in viral safety testing, facilitating the detection of adventitious agents and retrovirus-like particles (RVLPs) in biological products (e.g., mAbs, recombinant proteins, CGTs). TEM testing enables the detection of viral contaminants, including adventitious agents (e.g., parvovirus, polyomavirus, picornavirus, reovirus, adenovirus) and retroviral particles (e.g., type-A and type-C) in bulk harvest samples, cell banks, or virus seed stocks, as required by regulatory authorities. As outlined in the ICH guideline Q5A (R2), TEM should be utilized on the cell substrate used in biologics production and on unprocessed bulk harvest samples to detect and quantify RVLPs and other endogenous viruses or adventitious agents.
TEM is critical for testing and evaluating the viral safety of biotherapeutics and certain biological products, including mAbs, recombinant proteins, vaccines, cell therapy products (e.g., CAR-T cells, allogeneic cell therapy), and gene therapy vectors (e.g., AAVs, lentivirus and retrovirus). The potential for viral contamination in biotechnology products derived from cell lines is a significant concern due to the serious clinical consequences that could result. While there have been no reported cases of viral transmission through these products to date, maintaining rigorous viral safety is essential, as contamination may arise from the cell substrate itself or from adventitious viruses introduced during production. According to ICH guideline Q5A (R2), a comprehensive virus testing program must be implemented, alongside assessments of virus removal and/or inactivation achieved during the manufacturing process.
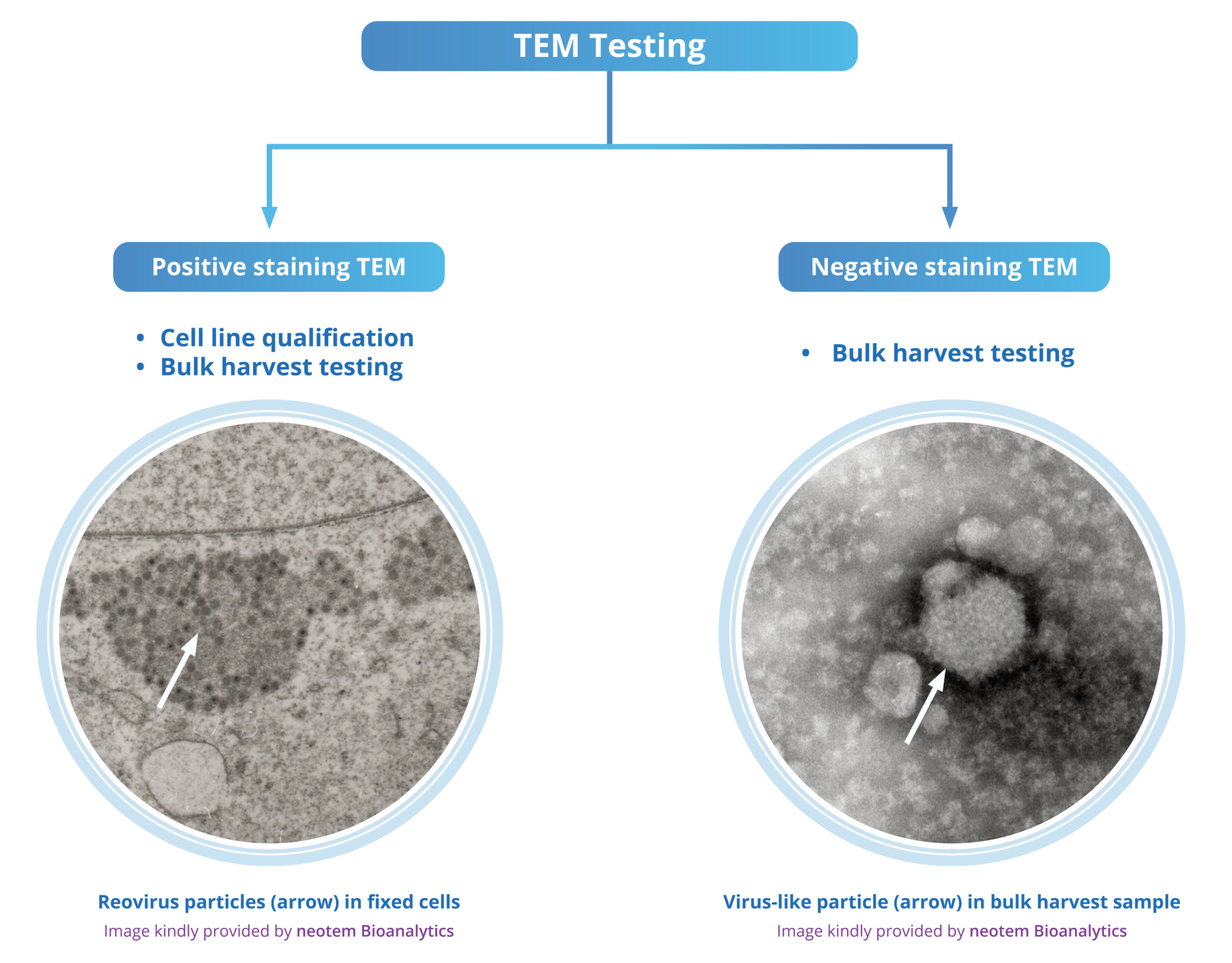
TEM for cell line qualification
As outlined in the ICH Q5A (R2) guideline, for cell line qualification, retrovirus testing must be performed on the MCB, and on cells cultured up to or beyond the LIVCA (also known as the End of Production Cell Bank, EOPCB) used in production. According to the guidelines, these tests include infectivity assays by direct inoculation or co-cultivation, assays for Reverse Transcriptase (RT) activity, and evaluation of particles by TEM. TEM is also capable of detecting other agents and is generally recommended for cell bank characterization.
Lipid droplet
Mitochondria
Positive staining TEM
Fixed Liver Cells
image kindly provided by neotem Bioanalytics
If a cell line is not known to produce retroviral particles, TEM must be performed on the cells, and a PCR-based RT assay (e.g., PERT assay) must be conducted on the clarified supernatant. RT activity can be associated with either infectious (type-C) or non-infectious (cytoplasmic type-A and type-R) retroviruses. If either TEM or RT results are positive, an assay to detect infectious retroviruses in permissible cells, including a human cell line and a sensitive readout assay for retrovirus detection must follow.
For cell lines known to produce retroviral particles (e.g., CHO, NS0, Sp2/0), RT activity is anticipated, eliminating the need for a PCR-based RT assay. TEM must be performed to examine the type of retroviral particles (e.g., type-A and type-C) present. This is essential for assessing whether the particles are infectious (type-C) or non-infectious (cytoplasmic type-A and type-R). Therefore, infectivity assays should be performed using relevant permissive cells (e.g., Mus dunni and SC-1 cells for rodent retroviruses) with sensitive readout assays for retrovirus detection (e.g., PERT assay, a Sarcoma-Positive, Leukemia-Negative (S+L-) assay, XC plaque assay). Retroviral testing results must be interpreted considering all available data. Learn more.
TEM for bulk harvest testing
TEM testing plays a crucial role in quantifying virus-like particles (VLPs) in unprocessed bulk harvests and is essential for identifying and assessing the presence of endogenous viruses, VLPs, during production. Endogenous viruses, such as retroviruses, may be acceptable if proper viral clearance evaluation procedures are followed.
According to ICH guideline Q5A (R2), TEM testing is used to investigate unprocessed cell-free culture supernatant. After centrifugation separates the cells from the test item, possible viruses or VLPs in the supernatant are concentrated by ultracentrifugation. C-type RVLPs released directly into the cell culture media are detected by TEM, along with any additional adventitious agents introduced during the production process.
Some cell lines, particularly those derived from rodents, insects, and avian species, are known to produce retroviral particles. For example, rodent-derived cell lines such as CHO, NS0, and SP2/0 often contain endogenous retrovirus particles or RVLPs. For these cell lines, it is essential that the manufacturing process effectively removes and/or inactivates such viral particles. Quantifying retroviral particles or RVLPs in bulk harvests is crucial for conducting viral clearance studies. Learn more.
The role of TEM testing in virus clearance evaluation
As stated in ICH guideline Q5A (R2), where only endogenous retroviruses or RVLPs are present, such as in rodent cell lines, the manufacturing process must be evaluated using specific “model” viruses like murine leukemia virus in addition to non-specific viral clearance evaluation. To obtain marketing authorization, data from at least three lots of purified bulk at the pilot plant or commercial scale must be provided. According to ICH guideline Q5A (R2), TEM is used to estimate the amount of virus in the unprocessed bulk, ensuring that the purification process substantially eliminates more virus than present in a single-dose equivalent of unprocessed bulk. Cell lines such as CHO, C127, BHK, and murine hybridoma (e.g., NS0 or SP2/0) have been widely used in drug production with no reported safety issues due to viral contamination. This approach may also be applicable to insect cell lines (e.g., Sf9) that produce extensively characterized endogenous RVLPs. For CHO cells e.g., a safety margin of < 10-4 particles/dose is considered acceptable for RVLPs for mAbs or other recombinant proteins if testing fails to identify the presence of such infectious retroviruses.
When no viruses, VLPs, or RVLPs other than the drug substance (e.g., viral vector particles) are detected in the cells or unprocessed bulk, virus removal and inactivation studies should be conducted using non-specific “model” viruses. If no RVLPs are detected and the result of the PERT assay is negative, an estimation of retroviral particles per dose is not required.
nsTEM and psTEM for bulk harvest testing and cell line qualification
Negative stain TEM (nsTEM) and positive stain TEM (psTEM) are two techniques used in TEM testing for biologics, including viral and retroviral characterization. nsTEM involves staining of samples with heavy metal salts, enhancing contrast by binding to the surface of biological specimens. This technique is commonly used in TEM testing for viruses to visualize viral particles, facilitating rapid detection and characterization. nsTEM is required by the regulatory authorities for bulk harvest testing. psTEM is used to enhance the visibility of specific structures within biological samples by staining them directly, rather than staining the background as in negative stain TEM. psTEM is required by the regulatory authorities for cell line qualification and provides enhanced resolution for detailed analysis of cellular structures.
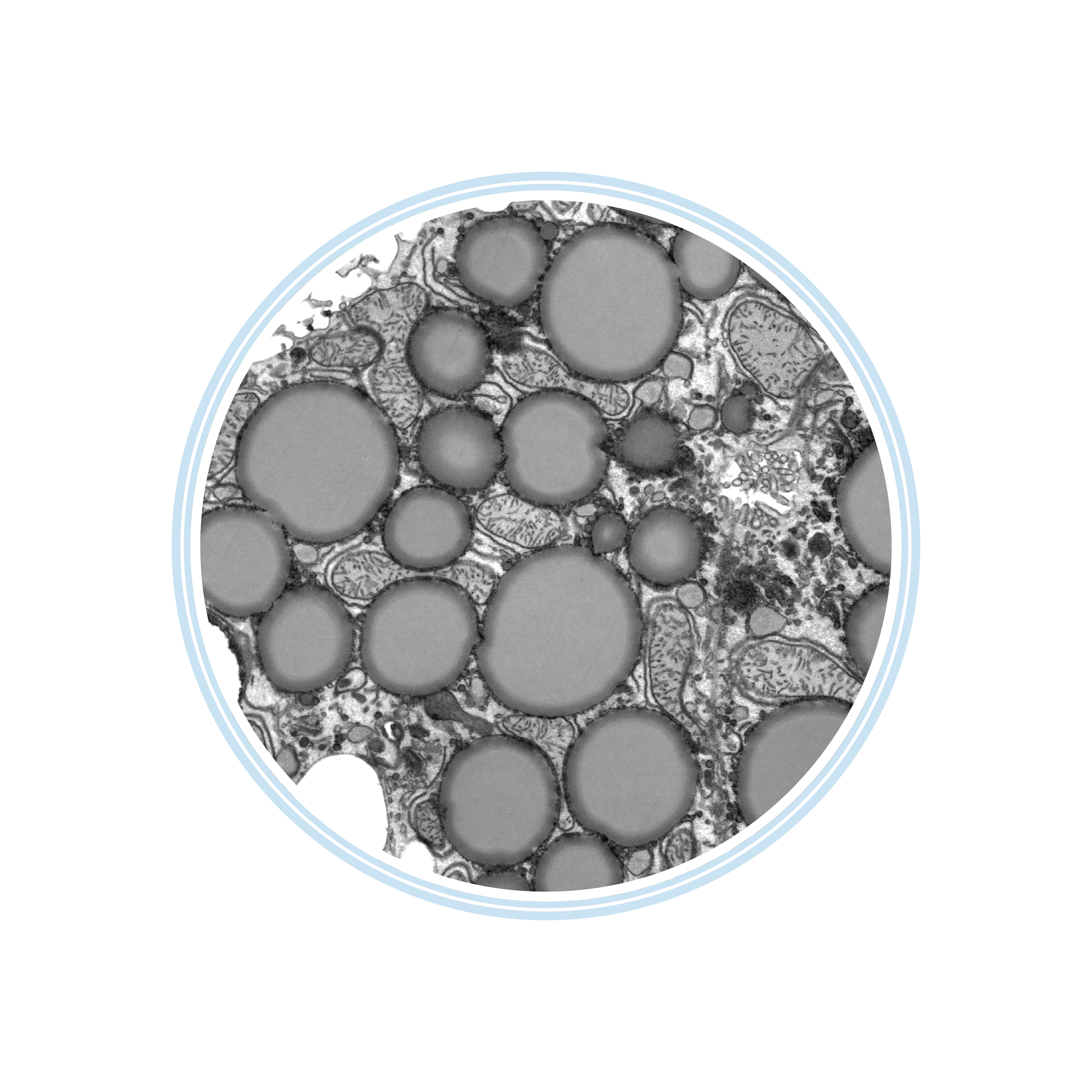
Virus-like particle in bulk harvest
Negative staining TEM
image kindly provided by neotem Bioanalytics
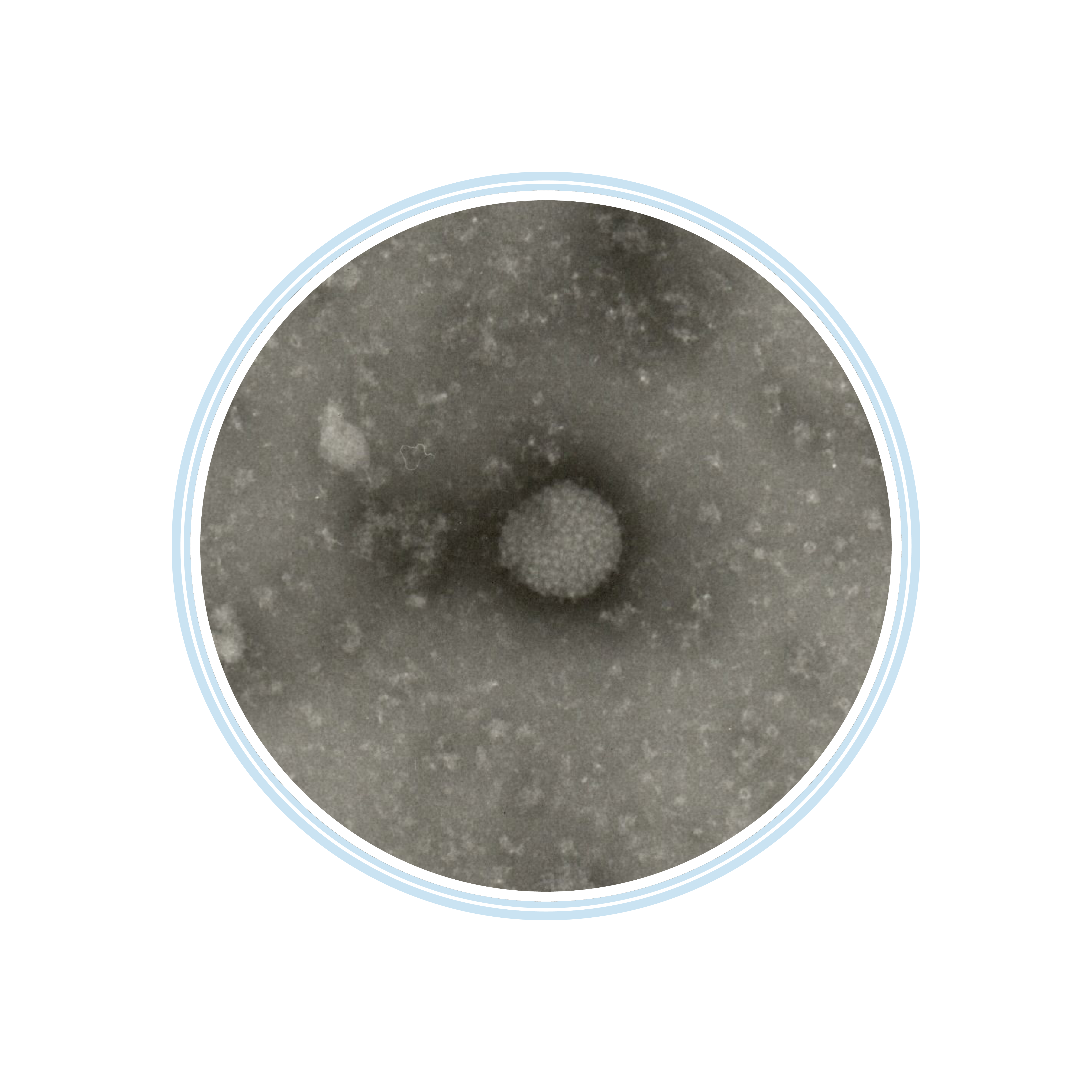
Flavivirus in fixed cells
Flavivirus in fixed cells
Flavivirus in fixed cells
Positive staining TEM
image kindly provided by neotem Bioanalytics
Key regulatory guidelines for TEM-based viral safety testing:
In the biopharmaceutical industry, ensuring the safety and efficacy of products derived from cell lines of human or animal origin is paramount. Key regulatory guidelines (e.g., ICH Q5A (R2), EMA’s gene therapy guideline, FDA guidance on cell substrates, etc.) provide comprehensive frameworks for viral safety testing. Adherence to these standards is essential for the successful commercialization of vaccines, CGTPs, ATMPs, and other recombinant products.
- ICH Guideline Q5A (R2) on Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin:
The guideline recommends the use of TEM for the detection and identification of viral contaminants (e.g., endogenous viruses, retroviruses, VLPs, adventitious agents) in biotechnology products. This guideline applies to products aiming for a Marketing Authorization Application (MAA) or a Biological License Application (BLA). - EMA Guideline on Virus Safety Evaluation of Biotechnological Investigational Medicinal Products:
The EMA guideline recommends TEM for evaluating the virus safety of biotechnological investigational medicinal products (IMPs). TEM is highlighted for its role in the detection of adventitious viruses and ensuring the absence of viral contamination in the production process. - EMA Guideline on Quality, Non-Clinical, and Clinical Aspects of Gene Therapy Medicinal Products:
The EMA guideline mandates the control of empty particles, aggregates, and replication-competent vectors in viral vector products. TEM is used to quantify the capsid population, verify capsid purity, and assess capsid integrity, thereby ensuring compliance with regulatory requirements for the commercialization of AAV products. - British Pharmacopoeia Guidance on Advanced Therapy Medicinal Products:
The British Pharmacopeia (BP) guidance recommends TEM for assessing the viral safety of ATMPs. TEM is suggested for its ability to detect and characterize viral particles, ensuring the safety and purity of these complex products. - FDA Guidance on Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs):
This FDA guidance recommends the use of TEM for the detection of viral contaminants in gene therapy products. TEM is emphasized for its role in the detailed morphological analysis of viral particles, helping to ensure the viral safety of gene therapy INDs. - FDA Guidance for Industry: Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications:
The FDA guidance advises using TEM to characterize and qualify cell substrates and other biological materials in viral vaccine production. TEM is recommended for its capability to visualize and identify endogenous and adventitious viral particles, ensuring the viral safety of vaccine products. - European Pharmacopoeia Chapter 5.1.7 on Viral Safety:
This chapter of the European Pharmacopoeia (Ph. Eur.) offers guidelines on viral safety testing requirements, including the use of TEM for identifying viral contaminants in gene therapy products. - European Pharmacopoeia Chapter 5.2.3 on Cell substrates for the production of vaccines for human use:
This chapter of the European Pharmacopoeia (Ph. Eur.) provides guidance on cell substrate testing for vaccine production, specifying the use of TEM testing for detecting retroviruses in the MCB and EOPC/ECB (cells at or beyond the maximum population doubling level used for production).
Let´s break down some FAQs!
What are virus-like particles?
VLPs (virus-like particles) are biological, noninfectious nanoparticles composed of the protective protein shell of a virus but lacking its viral genome. They mimic the structure of virus particles and are a specific class of viral subunits, providing a high immunogenic response.
As they are noninfectious and safe, they are often used in vaccine production (e.g., HPV, Hep B, influenza, malaria) to elicit a strong T- and B-cell-mediated immune response. The production of VLPs involves cell-based expression of viral envelope proteins and can be carried out in various systems, including mammalian cells (e.g., CHO), baculovirus/insect cells (e.g., SF9, Hi-5), microbial fermentation (e.g., E. coli, Pichia pastoris), and plants (e.g., tobacco).
How can VLP safety be ensured?
Though engineered as vaccines, VLPs can also arise naturally as byproducts of viral production, indicating potential contamination or inefficiencies. Ensuring the absence of VLPs in the final production is crucial for demonstrating the purity and safety of the production process. Engineered VLPs used as subunit vaccines or drug delivery systems must undergo rigorous viral safety testing. CHO cells e.g., contain partial or complete retroviral sequences, which can be expressed and co-purified with VLPs, contaminating the final product. According to ICH guideline Q5A (R2), TEM testing is recommended for evaluating VLPs to ensure they meet regulatory safety and purity standards.
Regulatory guidelines to follow:
- ICH Guideline Q5A (R2) on Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin.
- British Pharmacopoeia on Advanced Therapy Medicinal Products Guidance: Characterisation of the Capsid Particle Population in rAAV Products.
- EMA Guideline on the Quality, Non-clinical and Clinical Aspects of Gene Therapy Medicinal Products: EMA/CAT/80183/2014.
- FDA Guidance for Industry: Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs).
- FDA Guidance for Industry: Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications.
- European Pharmacopoeia:
Chapter 5.1.7. Viral Safety, 2023
If you’re interested in learning more, don’t hesitate to reach out
